Subalpine and Alpine Wildflowers and Pollinators of the North Cascades: Part 1

By Lauren Ridder, graduate student in the institute’s 14th cohort.
Nothing can quite prepare the hiker for the beauty of a subalpine meadow in full bloom. The contrast of the delicate flowers’ vibrant colors splashed across a backdrop of jagged peaks provides a moment for reflection and appreciation for the stark beauty of Cascadia. As the other senses kick in and notice is taken of fragrance on the breeze and a buzzing at the feet, the connections between plant, animal, insect, soil, water, and air become all too clear. Those relationships observed between plant and pollinator have been shaped by innumerable abiotic factors over millions of years. Wandering through a high mountain meadow provides a brief glimpse into the fascinating evolutionary history of wildflowers and their pollinators.
A Brief History of Plant Evolution
While observing the beautiful complexity of a wildflower, it can be hard to imagine the selection process that led from a single-celled photosynthetic organism living in a vast, watery world to a flower living high on the flanks of a mountain. Constantly changing environments guided the expansion of the plant kingdom and resulted in the development of vascular systems, seeds, and flowers (Visalli, 2014b). The green algal common ancestor found success over time in the colonization of land through embryo protection and the growth of a more solid tissue system.
This tissue system, or vascular system, transports water and nutrients throughout a plant, and is a more recent evolutionary development that allows for survival in harsher, drier environments.
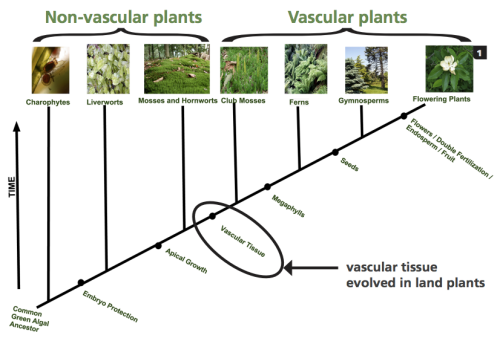
This cladogram of plant evolution shows the development of plant systems and the diversification of the plant kingdom over time (Guertin et al., 2015).
Without a vascular system, the nonvascular plants that exist today such as mosses, liverworts, hornworts, and algae are limited to shorelines, riverbanks, and other moist habitats. These more ancient plants do not have true roots, stems, or leaves though they eventually developed structures that are similar in appearance. Since water and nutrients are carried throughout the plant by osmosis and diffusion, which are processes that do not require energy, gravity limits the height of the plant. Proximity to water is vital, as nonvascular plants need water for both nutrient transport and reproduction. The vascular system developed more recently and uses active transport, which requires energy, to move water and nutrients though the tube-like xylem and phloem structures. Vascular plants are able to grow taller and larger with true roots, stems and leaves, and they can live farther from a water source. Modern members of this group include conifers, ferns, and all flowering plants (Guertin, Barnett, Denny, & Schaffer, 2015).
Further diversification of vascular plants comes in the form of their reproductive processes. The vascular plants living on land could no longer rely on water as a mode of transportation for their genetic material, so the plants that developed methods to protect and disperse this information in other ways were quickly selected for survival- thus the seed evolved. There are two groups of plants that produce a seed, the gymnosperms and the angiosperms, and the difference is in how the seed is packaged (Guertin et al., 2015). Gymnosperms, or ‘naked seed’ plants, are an ancient group of plants with unique reproductive processes that produce seeds that are protected by hard cones or fleshy, fruitlike coverings rather than an actual fruit. They have simple reproductive structures with either male or female reproductive organs, and their seeds are generally dispersed short distances by wind or gravity (Guertin et al., 2015). Examples of gymnosperms are conifers and gingkoes.
Angiosperms, or flowering plants, have a more recently evolved reproductive process. Their seeds are enclosed in a fruit, and their complex growth forms can support both male and female reproductive organs in a single flower or structure. Angiosperms also have more effective long-distance seed dispersal methods than angiosperms (Guertin et al., 2015). When identifying angiosperms, a good place to start is to discern whether the plant is a monocot or a dicot, meaning it has either one cotyledon, or seed leaf, or two cotyledons upon germination. Gymnosperms are multi-cotyledonous, with species germinating with anywhere between two and 24 seed leaves (Guertin et al., 2015). Monocots have plant parts that occur in three’s, for example three petals and six stamens. Their leaf veins are parallel and they have a fibrous root system. Members of the monocot group found in the North Cascades include grasses and lilies. All of the other vascular plants are dicots. Examples of North Cascadian dicots are roses and asters. Dicot plant parts occur in four’s and five’s, their leaf veins are netlike, and there is usually a taproot present in their root system (Visalli, “A Key”, n.d.). Distinguishing whether a plant is a monocot or a dicot takes only a couple minutes in the field and can help to narrow down which families to search through when trying to identify an unknown wildflower.
The Flower
Millions of years of evolution have led to the flower, which is the defining feature of angiosperms. These intricate structures come in thousands of different sizes and shapes, but all have but one purpose- reproduction. Flowers may look wildly different from biome to biome, but most have four whorls of flower parts: the sepals, petals, stamens, and pistils (Guertin et al., 2015). The outer whorls of the sepals and petals are the eye-catching parts of the flowers that sit atop the receptacle and contain the inner whorls of the stamens and pistils. The stamens are the male reproductive structures of the plant and are made up of a filament that holds up the anther, which produces the pollen. Ideally wind or pollinator will carry the pollen to the female reproductive structures, the pistils, or more specifically, the stigma, which is part of the pistil. The stigma is supported by the style that sits on the ovary. Upon fertilization of the ovary by an incoming pollen grain, the ovary develops into a seed with a protective casing that varies depending on the species.
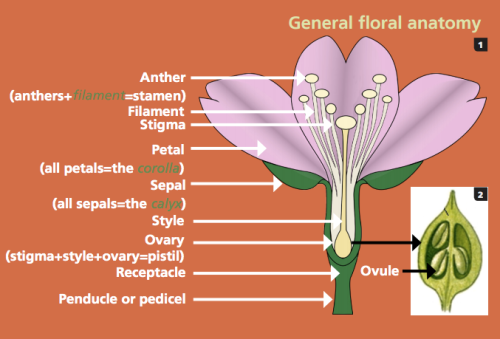
Diagram general floral anatomy (Guertin et al., 2015)
This oversimplified description of sexual reproduction is only half of the story. Angiosperms have been very successful at asexual reproduction and even tend to utilize that process more often than sexual reproduction to propagate their species. The harsh conditions of the alpine and subalpine environment often necessitate multiple methods of reproduction, and sexual reproduction is not always a viable option given the limited resources, even though it results in higher genetic diversity. The flower and its relationship with pollinators are key to sexual reproduction and therefore the plant’s successful adaptation over time to the challenges of the alpine (Visalli, 2014a). This relationship began about 150 million years ago, as insects were foraging among plants for food and happened to transfer pollen among plants in the process (Visalli, 2014a). Plants capitalized on this symbiotic relationship that reached “a level of complexity in ecosystems and on earth that had not previously existed. The partnership was so successful that flowering plants in a sense took over much of the terrestrial portion of the earth from the more ancient plants” (Visalli, 2014a, p.3).
This is part one of Lauren Ridder’s Natural History project. Stay tuned for part two!
References
Billings, W.D. (1974). Adaptations and Origins of Alpine Plants. Artic and Alpine Research, 6(2), 129-142.
Douglas, G.W., & Bliss, L.C. (1977). Alpine and High Subalpine Plant Communities of the North Cascades Range, Washington and British Columbia. Ecological Monographs, 47, 113-150.
Guertin, P., Barnett, L., Denny, E.G., Schaffer, S.N. (2015). USA National Phenology Network Botany Primer. USA-NPN Education and Engagement Series 2015-001. www.usanpn.org.
Meeuse, B., & Morris, S. (1984). The Sex Life of Flowers. New York, NY: Facts on File Publications.
National Park Service. (no date). Changing Plant Life. Natural Notes. Retrieved from http://www.nps.gov/noca/learn/nature/upload/NaturalNotes_07page3.pdf
Pojar, J., & MacKinnon, A. (1994). Plants of the Pacific Northwest Coast: Washington, Oregon, British Columbia, & Alaska. Vancouver, British Columbia, Canada: Lone Pine Publishing.
Pojar, J., & MacKinnon, A. (2013). Alpine Plants of the Northwest: Wyoming to Alaska. Edmonton, Alberta, Canada: Lone Pine Publishing.
Taylor, R.J., & Douglas, G.W. (1995). Mountain Plants of the Pacific Northwest. Missoula, MT: Mountain Press Publishing Company.
Visalli, D. (no date). A Key to the Common Flowing Plant Families of the Methow. The Methow Naturalist. http://methownaturalist.com/16- Key%20to%20Flowering%20Plants.PDF
Visalli, D. (2014a, July). Flora of the Methow: The Plants of the Methow in Their Evolutionary Context. Methow Naturalist. http://methownaturalist.com/7-2014-Flora%20of%20the%20Methow.pdf
Visalli, D. (2014b, Summer). Life in the High Country: A Naturalist’s Guide. The Methow Naturalist, 19(2), 1-3, 10-13. http://methownaturalist.com_2-MNv19n2web.pdf

